Classification, Labelling and Packaging (CLP) Regulation
The CLP Regulation (EC) No 1272/2008 ensures a high level of protection of health and the environment, as well as the free movement of substances, mixtures and articles through harmonized classification and labeling rules.
The CLP (Classification, Labelling and Packaging) Regulation, also known as Regulation (EC) No 1272/2008, is a European Union regulation that adopts the United Nations' Globally Harmonized System (GHS) for the classification and labelling of chemicals across all EU countries. This regulation ensures that chemicals are classified and labelled in a harmonized manner, making it easier for businesses and consumers to understand the hazards associated with chemicals.

Key Elements of CLP
- Harmonized Classification: Ensures consistent hazard assessment across the EU
- Self-Classification: Manufacturers, importers, and downstream users must classify substances and mixtures
- Labelling Requirements: Standardized format for communicating hazards
- Packaging Standards: Ensures safe containment and handling
Hazard Classification Categories
| Hazard Type | Categories | Examples |
|---|---|---|
| Physical Hazards | 16 classes | Explosives, Flammables, Oxidizers |
| Health Hazards | 10 classes | Acute Toxicity, Carcinogenicity |
| Environmental Hazards | 2 classes | Aquatic Toxicity, Ozone Depletion |
GHS Pictograms
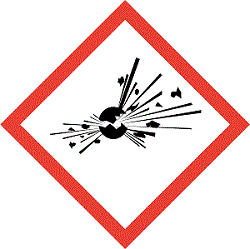

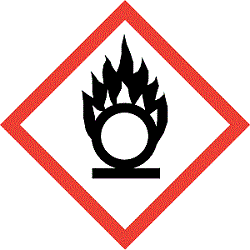
Labelling Elements
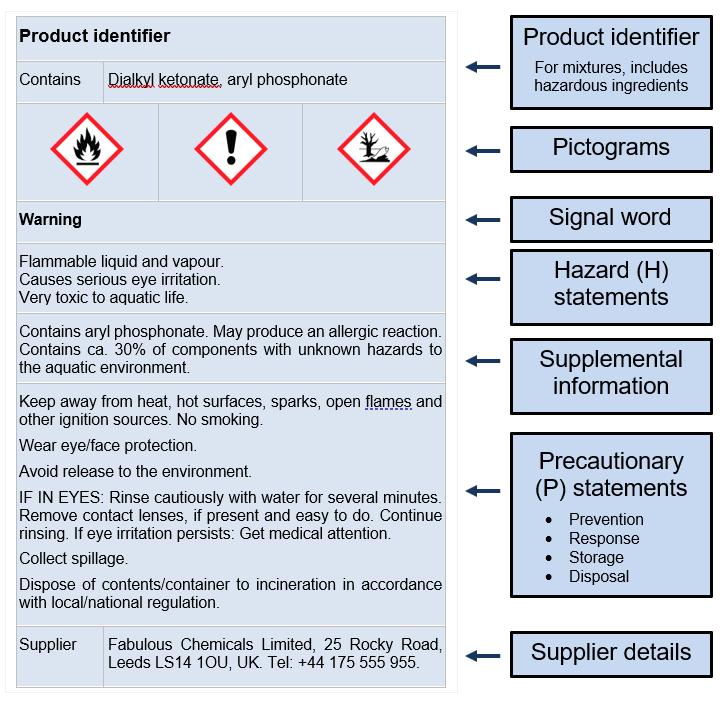
Required Label Elements:
- Product identifier
- Hazard pictograms
- Signal word (Danger/Warning)
- Hazard statements (H-statements)
- Precautionary statements (P-statements)
- Supplier information
- Nominal quantity
Mixture Classification
Important Considerations for Mixture Classification:
- Available data on the mixture itself
- Bridging principles
- Calculation methods
- Expert judgment
Implementation Timeline
| Date | Milestone | Requirements |
|---|---|---|
| 20 January 2009 | Entry into force | CLP published in Official Journal |
| 1 December 2010 | Substance deadline | Substances must be classified according to CLP |
| 1 June 2015 | Mixture deadline | Mixtures must be classified according to CLP |
Updates and Changes:
- Regular ATP updates
- New hazard classes consideration
- Harmonized classification updates
- Digital labeling developments
CLP Chemical Regulation Overview
The CLP (Classification, Labelling and Packaging) Regulation, also known as Regulation (EC) No 1272/2008, adopts the United Nations' Globally Harmonized System (GHS) for the classification and labelling of chemicals across all EU countries. This regulation ensures harmonized chemical classification and labelling, facilitating better understanding of chemical hazards for businesses and consumers.
CLP Annexes Explained
Annex I - Classification Criteria
Sets out criteria for classifying substances and mixtures in hazard classes and their differentiations. Includes provisions for physical, health, and environmental hazards classification.
Annex II - Special Rules
Contains special rules for classification and labelling of certain substances and mixtures with unique properties or characteristics.
Annex III - Hazard Communication
Lists hazard statements and precautionary statements required for labels and safety data sheets (SDSs), ensuring clear and consistent hazard communication.
Annex IV - Label Elements
Specifies pictograms, signal words, and hazard statements for labels and SDSs. Includes symbols representing different hazard types and severity indicators (Danger/Warning).
Annex V - Safety Data Sheets
Details the format and content requirements for Safety Data Sheets, ensuring comprehensive and consistent chemical safety information.
Annex VI - Harmonized Classification
Contains mandatory harmonized classification and labelling for certain hazardous substances, updated through yearly Adaptations to Technical Progress (ATP).
Annex VII - Language Translation
Provides guidance for translating hazard and precautionary statements into different languages, ensuring information accessibility across EU countries.
Connection with REACH
The CLP Regulation works in conjunction with the REACH Regulation (Registration, Evaluation, Authorisation, and Restriction of Chemicals). While CLP focuses on classification and labelling, REACH covers chemical registration, evaluation, authorization, and restrictions to protect human health and the environment.
Regulation (EU) 2020/878: Revised Requirements for EU Safety Data Sheets


Regulation (EU) 2020/878 amending the annex II of REACH regulation
On 25 Jun 2020, the European Commission published Regulation (EU) 2020/878 amending the annex II of REACH regulation. The annex II of REACH regulation sets the content and format requirements for chemical safety data sheets in the EU. The new regulation means that there will be revised requirements for SDSs for the EU market. In this article, we would like to give you a brief summary of what the main changes are.
General Requirements
The following two general requirements are new:
- The language used in the safety data sheet shall be simple, clear and precise, avoiding jargon, acronyms and abbreviations. Statements such as "may be dangerous", "no health effects", "safe under most conditions of use" or "harmless" or any other statements indicating that the substance or mixture is not hazardous or any other statements that are inconsistent with the classification of that substance or mixture shall not be used.
- All pages of a safety data sheet, including any annexes, shall be numbered and shall bear either an indication of the length of the safety data sheet (such as "page 1 of 3") or an indication whether there is a page following (such as "Continued on next page" or "End of safety data sheet").
Section 1: Identification of Substances/Mixtures
- Substances in nanoforms: If the safety data sheet pertains to one or more nanoforms, or substances that include nanoforms, this shall be indicated by using the word "nanoform" in product identifier in the section 1 of SDSs.
- Unique formula identifier: Where a mixture has a unique formula identifier (UFI) in accordance with section 5 of Part A of Annex VIII to Regulation (EC) No 1272/2008 and that UFI is indicated in the safety data sheet, then the UFI shall be provided in section 1.
Section 2: Hazard Identification
The communication through the supply chain for endocrine disruptors will be improved. Not only information on whether the substance meets the criteria for persistent, bioaccumulative and toxic or very persistent and very bioaccumulative should be given in section 2.3 other hazards, information on whether the substance was included in the list established in accordance with Article 59(1) for having endocrine disrupting properties, and whether the substance is a substance identified as having endocrine disrupting properties.
For a mixture, information shall be provided for each such substance that is present in the mixture at a concentration equal to or greater than 0,1 % by weight.
Enforcement
The new regulation comes into force on 1 Jan 2021. Existing SDSs can be used until 31 December 2022.
Reference
Commission Regulation (EU) 2020/878 of 18 June 2020 amending Annex II to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)
Official Journal of the European Union - Regulation (EU) 2020/878